This all-in-one online Bond Order Calculator performs calculations using a formula that relates the number of bonding and antibonding electrons in molecular orbitals to the bond order. You can enter the values of any two known parameters in the input fields of this calculator and find the missing parameter.
Bond Order in Molecular Orbital Theory
In chemistry, molecular orbital theory is a way of explaining how atoms combine to form molecules. It is based on the idea that atoms can share electrons to form covalent bonds. A covalent bond is a chemical bond in which two atoms share a pair of electrons.
Bond order is a term used in molecular orbital theory to describe the strength of a chemical bond. It represents the number of chemical bonds between a pair of atoms. A higher bond order indicates a stronger bond.
According to molecular orbital theory, the electrons in a molecule are distributed among molecular orbitals, which are formed by combining the atomic orbitals of the individual atoms. These molecular orbitals can be either bonding or antibonding, depending on the phase relationship between the atomic orbitals.
Bonding orbitals are formed when the phase of the atomic orbitals of the two atoms is the same. In other words, the wave functions of the two atomic orbitals add constructively, resulting in a molecular orbital with increased electron density between the atoms and a lower energy than the original atomic orbitals. Electrons in bonding orbitals contribute to the stability of the molecule by forming a covalent bond.
On the other hand, antibonding orbitals are formed when the phase of the atomic orbitals of the two atoms is opposite. In this case, the wave functions of the two atomic orbitals cancel each other out in the space between the two atoms, resulting in a molecular orbital with decreased electron density between the atoms and a higher energy than the original atomic orbitals. Electrons in antibonding orbitals do not contribute to the stability of the molecule and can actually weaken the bond between the two atoms.
Bond Order Formula
The bond order can be calculated using various formulas, depending on the type of molecule being studied. For diatomic molecules (molecules with only two atoms), the bond order can be calculated using the following formula:
This formula takes into account the difference between the number of electrons in bonding and anti-bonding orbitals, divided by two. A higher bond order indicates a stronger bond, while a lower bond order suggests a weaker bond. A bond order of zero implies that there is no bond between the atoms.
Bond order is directly related to the strength and stability of a chemical bond. A higher bond order indicates a stronger bond, which requires more energy to break. This information is useful in predicting the reactivity of molecules and their potential to undergo chemical reactions.
Bond order is inversely proportional to the bond length between two atoms in a molecule. A higher bond order results in a shorter bond length, while a lower bond order leads to a longer bond length. This relationship helps chemists predict the geometry and structure of molecules.
For diatomic molecules, the bond order can be calculated using the molecular orbital diagram, which shows the distribution of electrons in various molecular orbitals. Consider two simple examples of hydrogen and helium molecules (the pictures below are from Wikipedia).
Example1. Hydrogen molecule – H2.
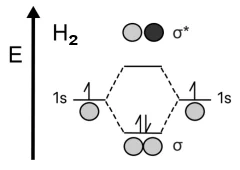
In the classic example of H2, two individual hydrogen atoms have the same atomic orbitals. When creating an H2 hydrogen molecule, the individual valence orbitals, \(1s\), are either added to form the bonding orbital \(\sigma_{1s}\) where the electron density is maximum between the nuclei of the atoms, or are subtracted, which gives the antibonding orbital \(\sigma^*_{1s}\) where the electron density between the nuclei of the two atoms is at a minimum.
Bonding orbitals result in a more stable state of the system with a lower energy than when the two hydrogen atoms remain monatomic. Antibonding orbitals are less stable because, due to the very low electron density between the two nuclei, these equally charged nuclei repel each other. Therefore, it will take more energy to hold two atoms together if they are connected by an antibonding orbital.
All electrons from the valence \(1s\) shell of hydrogen atoms come together to fill the bonding orbital in accordance with the Pauli principle. So, hydrogen prefers to exist as a diatomic rather than a monatomic molecule.
To calculate the bond order in a hydrogen molecule, we plug the number of electrons in bonding orbitals (2) and the number of electrons in anti-bonding orbitals (0) into the above formula. As a result, we get: Bond order = (2 – 0) / 2 = 1.
Example2. Helium molecule – He2.
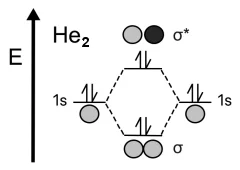
Another simple example is helium He. An atom of this element contains two electrons in the valence \(1s\) shell. When two helium atoms approach each other, as in the case of hydrogen, two molecular orbitals are formed: bonding and antibonding.
When two atomic orbitals combine, they first fill the bonding orbital with two electrons, but, unlike hydrogen, two more electrons remain, which must then go to the antibonding orbital. The effect of the antibonding orbital negates the stabilizing effect provided by the bonding orbital. If we calculate the bond order of dihelium using our formula, we get the following result: Bond order = (2 – 2) / 2 = 0. This is why helium prefers to be monatomic rather than diatomic.
The examples given here are quite simple and the calculations do not cause problems. However, in more complex cases it is much more convenient to use our online Bond Order Calculator.
Application in Chemistry
The concept of bond order is used in many areas of chemistry, including predicting the reactivity and stability of molecules, understanding the properties of materials, and designing new molecules with specific properties. For example, chemists may use bond order calculations to predict the strength of materials such as polymers, which are made up of long chains of covalently bonded molecules.
Bond order can also be used to predict the magnetic properties of molecules. Molecules with unpaired electrons, such as those with odd numbers of electrons in antibonding orbitals, are generally paramagnetic, meaning they are attracted to a magnetic field. In contrast, molecules with all electrons paired, such as those with even numbers of electrons in antibonding orbitals, are diamagnetic, meaning they are not attracted to a magnetic field.
Related calculators
Check out our other chemistry calculators such as Average Atomic Mass Calculator or Molecular Formula Calculator.