This online pH calculator is designed to determine the pH of an aqueous solution of a given chemical compound. You can select any acid or base from the list of chemicals, or use a known value for the dissociation constant Ka or Kb. The concentration of a substance can be specified either in moles or in units of mass per unit volume.
pH Scale
In chemistry, \(pH\) (denoting “potential of hydrogen” or “power of hydrogen”) is a quantitative measure used to specify the acidity or basicity of an aqueous solution.
\(pH\) is defined as the negative decimal logarithm of the hydrogen ion activity, \(a{_{H}}^{+}\), in a solution:
$$pH=-log_{10}(a{_{H}}^{+}).$$
The hydrogen ion activity, in turn, depends on the concentration of hydrogen ions, which in dilute solution can be expressed by the following formula:
$$a{_{H}}^{+}=[{H}^{+}]/c_{0},$$
where
\([{H}^{+}]\) is the concentration of hydrogen ions,
\(c_{0}\) is the standard state concentration \(c_{0}\) = 1 mol/L.
So, \(a{_{H}}^{+}\) is a dimensionless number whose logarithm can be defined. In practice, the following formula is used to calculate \(pH\):
$$pH=-log_{10}([{H}^{+}]),$$
where \([{H}^{+}]\) is considered to be given in units of mol / L.
In pure (neutral) water at 25 °C the concentration of the hydrogen ion is 10−7 mol / L. This gives us a \(pH\) of water equal to 7. The neutral value of the \(pH\) depends on the temperature – being lower than 7 if the temperature increases.
A solution with a \(pH\) less than 7 is considered acidic; a solution with a \(pH\) greater than 7 is considered basic, or alkaline. \(pH\) ordinarily ranges between 0 and 14. The \(pH\) value can be less than 0 for very strong acids, or greater than 14 for very strong bases.
Similarly, we can define \(pOH\) as a measure of the concentration of hydroxide ions, \(O{H}^{-}\):
$$pOH=-log_{10}([O{H}^{-}]).$$
Due to the fact that the concentrations of \({H}^{+}\) and \(O{H}^{-}\) in pure water are equal, it is easy to show that at 25°C the following formula is valid:
$$pH + pOH = 14.$$
The \(pH\) values found in nature and in everyday life are presented in the following \(pH\) scale illustration.
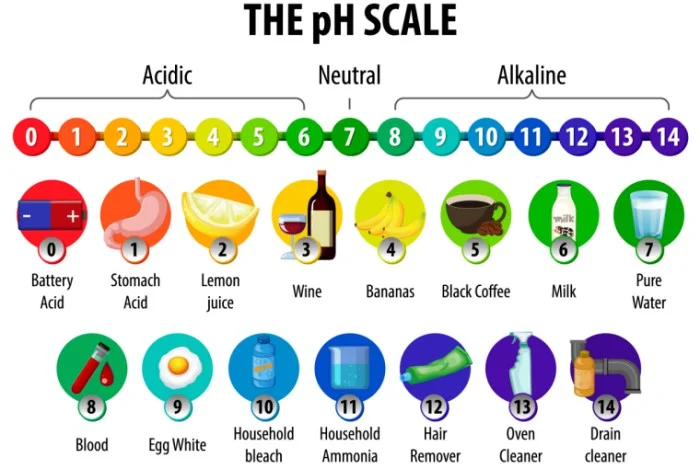
How to Calculate pH
The calculation of a \(pH\) value from the molar concentration of a solution is different for strong and weak acids and bases.
Strong acids and bases
Strong acids and bases are chemicals that under normal circumstances dissociate almost completely in water. This means that the concentration of hydrogen ions in acidic solution can be taken to be equal to the concentration of the acid. Similarly, the concentration of hydroxide ions in an alkaline solution can be taken to be equal to the concentration of the alkali.
In this case, we can use the above formulas for \(pH\) and \(pOH\) by simply substituting acid and base concentrations instead of \([{H}^{+}]\) and \([O{H}^{-}]\), respectively.
Only seven acids and only five water-soluble bases are considered strong.
Weak acids and bases
Weak acids and bases only partially dissociate in water. This complicates the calculation of \(pH\). In this case, to calculate the concentration of ions \([{H}^{+}]\), you will also need the dissociation constant. For an acid, this constant is determined as follows:
$$K_{a}= \frac{[{H}^{+}][{A}^{-}]}{[HA]},$$
where
\([{A}^{-}]\) is the concentration of anions,
\([HA]\) is the concentration of undissociated compound.
The dissociation constant shows the equilibrium condition for the aqueous solution:
$$HA \rightleftharpoons H^{+} + A^{-}.$$
It is easy to show that, knowing the dissociation constant \(K_{a}\) and the molar concentration of acid \(C_{a}\), the following equation can be obtained for \([{H}^{+}]\):
$$[H^{+}]^2 + K_{a} [H^{+}] – K_{a}C_{a} = 0.$$
Solution of this quadratic equation gives the hydrogen ion concentration and hence the \(pH\) value.
The same method is applied to solutions of bases, only the base dissociation constant \(K_{b}\) is used and \(pOH\) is calculated first.
It should be noted that some polybasic (or polyprotic) acids can donate more than one proton per molecule into solution. However, due to molecular forces, the \(K_{a}\) value for each next proton decreases by several orders of magnitude. So, we consider in our pH Calculator the dissociation constant \(K_{a}\) for donation of only one proton. The values of the constants \(K_{a}\) and \(K_{b}\) are taken from this source.
Why is pH Important?
In many cases it is important to know the pH of a solution because it can have a significant impact on its properties and behavior. Here are some real-life examples:
• Food and drink. The pH of food and drink can affect its taste and stability. For example, the pH of coffee is around 5 which contributes to its acidity and bitterness. The pH of milk is around 6.5 which contributes to its neutral taste.
• Aquariums. The pH of water in an aquarium can impact the health and well-being of the fish and other aquatic life. Different species of fish have different pH requirements, and maintaining the proper pH can help prevent disease and promote healthy growth.
• Industrial processes. The pH of solutions used in industrial processes can affect the efficiency and effectiveness of the process. For example, certain pH values of water used in boilers can lead to scaling and corrosion and, thus, influence its ability to transfer heat. The pH of solutions used in metal plating can affect the quality of the finished product.
• Medicine. The pH of some medications can affect how they are absorbed and metabolized by the body. For example, some medications are designed to be taken with a specific pH in order to optimize their effectiveness.
• Cleaning and hygiene. The pH of cleaning solutions can affect their ability to remove dirt and stains. For example, acidic solutions are often used to remove mineral deposits while basic solutions are often used to remove grease and oil.
Related calculators
Check out our other chemistry calculators such as Dilution Calculator or Molecular Weight Calculator.
Illustration created by brgfx / Freepik.